Author: Brittany Hartman, DO Emergency Medicine Resident, PGY II
Fellow: Richard Chen, MD, Medical Toxicology / Emergency Medicine Attending
Faculty: David Goldberger, MD, Medical Toxicology / Emergency Medicine Attending
The Case
A 22-year-old pre-operative male-to-female transgender patient with a history of PTSD and depression presents to a Midwest ED following a suicide attempt. The patient states she had ingested one ground-up “pong-pong” seed approximately 7 hours prior to arrival, and states she purchased this seed online. She is now complaining of chest pain and “feeling weird.”
Vitals: HR 53, RR 15, BP 127/76, T 36.8C, SpO2 99% on RA
Physical exam shows GCS 15. Pupils are 3mm and reactive bilaterally; mucous membranes are moist. Cardiac exam reveals no murmurs but is notable for bradycardia. Respiratory and abdominal exam are within normal limits. Skin was warm and dry. Neurologic exam is also unremarkable with no sensory deficits, no clonus, no rigidity, and normal reflexes throughout.
Given your concern for “pong-pong” ingestion, you reach out to your local poison control center to speak to a toxicologist. They inform you that a “pong-pong” seed is a type of cardiac glycoside and advise you on what to look for and how to begin treatment for this toxic ingestion.
Learning Point 1
“Pong-Pong” or Cerbera odollam is a cardiotoxic plant of the Apocynaceae family, most commonly found in South India, Madagascar, Southeast Asia and Australia. It has been used as an ornamental tree in many non-endemic areas, including Hawaii, as the bright green fruits turn bright red at maturity, proving it to be a colorful, ornamental hedge plant. The flower produced by the tree before the fruiting body is said to have a pleasant odor, and thus it lends itself well to a decorative addition to a garden or greenhouse. It is easily obtained as seeds on Ebay and the dried seeds can be sold as home décor.
This beautiful plant, however, houses an incredibly toxic kernel at the core of the fruiting body. The kernel contains a cardioactive glycoside called cerberin. Although all parts of the plant are toxic, the fruiting kernel itself is the most toxic, causing cardiotoxicity with as little as half a seed. The kernel, as well as other parts of the tree are responsible for approximately 50% of the plant poisoning cases and up to 10% of the total poisoning cases in Kerala, India, where it is commonly used for both homicide and suicide.


Toxicokinetics of Cardioactive Steroids
Although a common toxin in much of Southeast Asia and India, the toxicokinetics of Cerberin are not well studied. They are thought to be similar to digoxin, a common cardioactive glycoside used in treatment of heart failure and atrial fibrillation. Digoxin is generally rapidly distributed from the blood to peripheral tissues, with a peak serum concentration of oral digoxin at approximately 1-2 hours after administration. Digoxin has a large volume of distribution, tending to distribute to skeletal muscle and has a low protein binding in the serum.
Digoxin is almost entirely cleared renally, with the remaining metabolism performed by the liver. The kidneys excrete between 60-80% of digoxin unchanged. The half-life of digoxin varies widely based on dose and renal clearance, but it appears to be between 13-48 hours in people with normal renal function and up to 6 days in those with renal failure or compromised function. Elevated serum concentrations of digoxin result in greater renal clearance before distribution to the tissues.
The Case Continues
You obtain an initial EKG which shows a second-degree heart block in 2:1 pattern as follows:
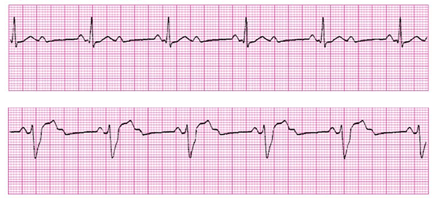
The patient’s bloodwork is notable for potassium of 5.2 and negative troponin. Remainder of serum chemistry panel and CBC within normal limits. Her urine drug screen was negative and her salicylate, acetaminophen, and ethanol levels were undetectable.
Learning Point 2
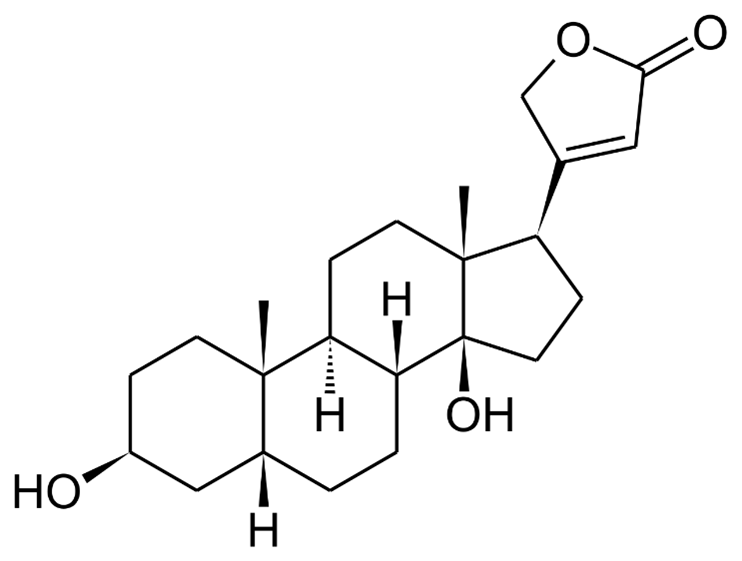
Mechanism of Action: Cardioactive glycosides directly inhibit the sodium-potassium-ATPase pump. The sodium-potassium ATPase pump maintains the cardiac membrane potential by maintaining the sodium concentration gradient across the cell membrane. Blocking this pump increases the sodium concentration inside the myocyte, disrupting the normal gradient. The disruption of the gradient leads to dysfunction of other cellular transporters, particularly the sodium-calcium exchange (NCX) transporter. This transporter functions to keep the concentration of intracellular calcium low by exchanging calcium for sodium. This results in increased calcium levels in the myocyte. The elevated intracellular calcium leads to a larger calcium induced calcium release from the endoplasmic reticulum. This ultimately leads to stronger contraction of myosin and increased ionotropy.
In toxicity, intracellular calcium increases so much that it affects the resting membrane potential, bringing it closer to zero. This change makes the myocyte more sensitive to depolarization, automaticity and predisposes to dysrhythmias. Cerberin is also thought to increase vagal tone in both the SA and AV nodes, which leads to increased refractoriness and decreased conduction velocity, further increasing the likelihood of ventricular dysrhythmia. Classic EKG changes include QT interval shortening and scooped ST segment, or “The digoxin effect.” This finding does not signify toxicity, but is often found in patients chronically taking digoxin. In acute overdose, PVCs often are the initial EKG finding, due to increased automaticity. Various degrees of heart block can be seen as well as ventricular dysrhythmias.

Figure illustrating effects of digoxin on cardiac myocytes. Source: Chapter 62 – Cardioactive Steroids. Goldfrank’s Toxicologic Emergencies 11th edition
Learning Point 3
Clinical presentation and management
Acute Toxicity:
- Noncardiac Manifestations
- Nausea, vomiting, abdominal pain
- Lethargy, confusion, weakness
- Thrombocytopenia (more likely with Cerberin toxicity)
- Electrolyte abnormalities
- Hyperkalemia (>5.5 mEq/L associated with 100% mortality in one study in acute toxicity)
- Cardiac manifestations
- Nearly any dysrhythmia, although bidirectional ventricular tachycardia is considered pathognomonic
- Bradydysrhythmias common due to increased vagal tone at the SA and AV nodes
- Unlikely to see supraventricular tachycardias due to AV nodal blockade
Diagnostics
- Diagnosis of non-digoxin cardiac glycoside toxicity is done with history suggesting exposure and signs of cardiotoxicity
- An EKG and a potassium level are helpful in the diagnosis
- Obtaining a digoxin level may be helpful. This assay normally measures serum concentration of digoxin but can detect presence of other cardiac glycosides due to cross-reactivity. Because the assay is specific to digoxin, the resulting level can only be interpreted as a qualitative test.
- There is literature that suggests minimal cross-reactivity between the digoxin assay and cerberin. As a result, an undetectable digoxin level with concern for cerberin exposure cannot be used to rule out cerberin toxicity
- Cerberin levels can be obtained but are typically not readily available in most hospital laboratories and will require send out testing. As a result, acute management will not be influenced by Cerberin serum concentrations.
Treatment
- Supportive treatments including IV fluids, vasopressors
- Bradycardia: atropine, pacing
- Arrhythmias: ACLS pathway including cardioversion
- Hyperkalemia: If digoxin immune fab are available, this should be first line treatment as neutralization of cerberin should correct potassium. If not available, or if patient is hemodynamically unstable, treatment with calcium should be pursued. Other usual temporizing treatments, such as sodium bicarbonate, insulin (with dextrose), and nebulized beta-agonists can also be used
- Digoxin immune fab (DIF) may reduce cardiotoxic effects of Cerberin. DIF are mixed anti-digoxin immunoglobulin fragments that are obtained from healthy sheep. These immunoglobulins have a high affinity for digoxin. DIF binding to digoxin molecules reduces their availability for binding to the ATPase, thus reducing the toxic effects of the digoxin molecule itself. There is cross-reactivity between non-digoxin cardiac glycosides and digoxin specific antibodies. As a result, DIF likely can treat toxicity from Cerberin. However, the cross-reactivity is limited, and often 20 or more vials are needed to reduce cardiotoxicity from Cerberin, and in fact, DIF may not be effective in reversing the effects of Cerberin if the ingested dose was significant.
Case Resolution
Patient was administered 5 vials of digoxin immune fab and given supportive care with serial EKGs.
After administration of Digoxin immune fab, the patient had improvement in her heart block (see EKG) with return to sinus rhythm with first-degree AV block and persistent ST-segment depression and biphasic T-waves.
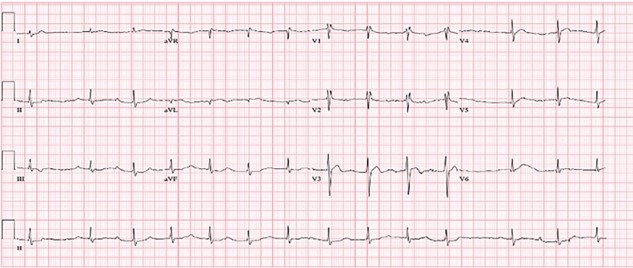
However…
2 hours into the ED course, a repeat EKG was performed:
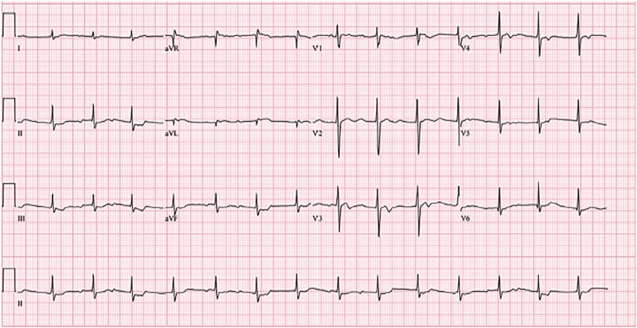
You read the EKG as a complete heart block. You gave 5 more vials of Digoxin immune fab and a repeat serum K was 5.7 mEq/L. Thirty minutes later, the patient became acutely unresponsive, and was found to be in a PEA arrest. Five more vials of Digoxin immune fab were administered. The patient was then intubated and given 10 more vials of Digoxin immune fab. Despite your best efforts, the patient failed to achieve ROSC and expired after a prolonged resuscitation effort. Postmortem forensic toxicology testing identified Cerberin in the patient’s serum.
Take Home Points
1) Cerberin, a cardiac glycoside with a similar toxic profile to digoxin, is found in the plant Cerbera odollam, and is found throughout Southeast Asia, India, and easily ordered online
2) Cerberin works by inhibiting the sodium-potassium ATPase pump. This results in increased calcium concentration in the myocyte. In toxicity, this excess calcium increases the likelihood of depolarization of the myocyte by bringing the resting membrane potential closer to zero
3) Cerberin toxicity manifests primarily with cardiotoxic effects, but patients can also have nausea, vomiting, abdominal pain, hyperkalemia, and thrombocytopenia.
4) Cardiac manifestations of toxicity typically include PVCs, bradycardia and ventricular dysrhythmias
5) Treatment is primarily supportive care, although digoxin immune Fab may be beneficial. Doses for reversal of Cerberin toxicity will be far higher than that required for treatment of digoxin toxicity
References
Bernshteyn, M., Adams, S. H., & Gada, K. (2020). A Case of Attempted Suicide by Cerbera odollam Seed Ingestion. Case Reports in Critical Care, 2020, 1–5. https://doi.org/10.1155/2020/7367191
Botelho, A. F., Pierezan, F., Soto-Blanco, B., & Melo, M. M. (2019). A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon, 158, 63–68. https://doi.org/10.1016/j.toxicon.2018.11.429
Design2Gether. (n.d.). Pong Pong seed /pack 5 Seed. Etsy. https://www.etsy.com/listing/1036478735/pong-pong-seed-pack-5-seed?gpla=1&gao=1&&utm_source=google&utm_medium=cpc&utm_campaign=shopping_us_a-craft_supplies_and_tools-floral_and_garden_supplies-greenery_and_gardening-other_greenery-seeds&utm_custom1=_k_EAIaIQobChMIiL6OjOSa8QIVQaCGCh1F7AhkEAQYASABEgJy8vD_BwE_k_&utm_content=go_2063077358_76452861975_367965824289_aud-1185363470307%3Apla-320728462966_c__1036478735_12768591&utm_custom2=2063077358&gclid=EAIaIQobChMIiL6OjOSa8QIVQaCGCh1F7AhkEAQYASABEgJy8vD_BwE.
Eva. (2016, February 24). A surprising tropical plant : Cerbera odollam / Suicide Tree. Culture Acre. http://www.culture-acre.com/surprising-tropical-plant-cerbera-odollam-suicide-tree/.
Fok, H., Victor, P., Bradberry, S., & Eddleston, M. (2017). Novel methods of self-poisoning: repeated cardenolide poisoning after accessing Cerbera odollam seeds via the internet. Clinical Toxicology, 56(4), 304–306. https://doi.org/10.1080/15563650.2017.1369543
Gaillard, Y., Krishnamoorthy, A., & Bevalot, F. (2004). Cerbera odollam: a ‘suicide tree’ and cause of death in the state of Kerala, India. Journal of Ethnopharmacology, 95(2-3), 123–126. https://doi.org/10.1016/j.jep.2004.08.004
Goldfrank, L. R., & Flomenbaum, N. (2006). In Goldfrank’s toxicologic emergencies (11th ed.). essay, McGraw-Hill.
Kiang, L. (2020). Suicide from a Cerbera odollam kernel. Archives of Medical Case Reports and Case Study, 3(1), 01–05. https://doi.org/10.31579/2692-9392/007
Levine, M. (2019, January 17). Digoxin overdose, Cardiac glycoside toxicity, digoxin toxicity, digoxin poisoning. Cancer Therapy Advisor. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/critical-care-medicine/digoxin-overdose-cardiac-glycoside-toxicity-digoxin-toxicity-digoxin-poisoning/.
Menezes, R. G., Usman, M. S., Hussain, S. A., Madadin, M., Siddiqi, T. J., Fatima, H., Ram, P., Pasha, S. B., Senthilkumaran, S., Fatima, T. Q., & Luis, S. A. (2018). Cerbera odollam toxicity: A review. Journal of Forensic and Legal Medicine, 58, 113–116. https://doi.org/10.1016/j.jflm.2018.05.007
Menon, M. S., Kumar, P., & Jayachandran, C. I. (2016). Clinical profile and management of poisoning with suicide tree: An observational study. Heart Views, 17(4), 136. https://doi.org/10.4103/1995-705x.201783
Misek, R., Allen, G., LeComte, V., & Mazur, N. (2018). Fatality Following Intentional Ingestion of Cerbera odollam Seeds. Clinical Practice and Cases in Emergency Medicine, 2(3), 223–226. https://doi.org/10.5811/cpcem.2018.5.38345
Randerson, J. (2004, November 24). ‘Suicide tree’ toxin is ‘perfect’ murder weapon. NewScientist.
Renymol, B., Palappallil, D., & Ambili, N. (2018). Study on clinical profile and predictors of mortality in Cerbera odollam poisoning. Indian Journal of Critical Care Medicine, 22(6), 431–434. https://doi.org/10.4103/ijccm.ijccm_469_17
The Toxic Tale of the Murder Tree. Commonplace Fun Facts. (2019, October 4). https://commonplacefacts.wordpress.com/2019/10/04/the-toxic-tale-of-the-murder-tree/.
Usui, K., Fujita, Y., Seki, A., Kamijo, Y., & Funayama, M. (n.d.). Analysis of cardiac glycosides in “suicide tree” kernel . 16th Annual Scientific Congress – 2017. http://www.apamt.org/wp-content/uploads/2018/01/16th_EPoster_36.pdf. Wermuth, M. E., Vohra, R., Bowman, N., Furbee, R. B., & Rusyniak, D. E. (2018). Cardiac Toxicity from Intentional Ingestion of Pong-Pong Seeds (Cerbera Odollam). The Journal of Emergency Medicine, 55(4), 507–511. https://doi.org/10.1016/j.jemermed.2018.05.021